For the Te structure use the periodic table to find the total number of valence electrons for the Te molecule. Once we know how many valence electrons there
TeBr4 Lewis Structure in 5 Steps (With Images)
Jun 23, 2023TeBr4 lewis structure has a Tellurium atom (Te) at the center which is surrounded by four Bromine atoms (Br). There are 4 single bonds between the Tellurium atom (Te) and each Bromine atom (Br). There is 1 lone pair on the Tellurium atom (Te) and 3 lone pairs on all the four Bromine atoms (Br).

Source Image: anyrgb.com
Download Image
Mar 13, 2023Step #1: Calculate the total number of valence electrons Here, the given molecule is TeBr4. In order to draw the lewis structure of TeBr4, first of all you have to find the total number of valence electrons present in the TeBr4 molecule. (Valence electrons are the number of electrons present in the outermost shell of an atom).

Source Image: academic-accelerator.com
Download Image
Tellurium tetrabromide | TeBr4 | CID 82311 – PubChem Tellurium tetrabromide ( Te Br 4) is an inorganic chemical compound. It has a similar tetrameric structure to TeCl 4. [3] It can be made by reacting bromine and tellurium. [4] In the vapour TeBr 4 dissociates: [3] TeBr 4 → TeBr 2 + Br 2 It is a conductor when molten, dissociating into the ions TeBr 3+ and Br −.

Source Image: dreamstime.com
Download Image
Draw The Lewis Structure For The Tellurium Tetrabromide Molecule
Tellurium tetrabromide ( Te Br 4) is an inorganic chemical compound. It has a similar tetrameric structure to TeCl 4. [3] It can be made by reacting bromine and tellurium. [4] In the vapour TeBr 4 dissociates: [3] TeBr 4 → TeBr 2 + Br 2 It is a conductor when molten, dissociating into the ions TeBr 3+ and Br −. Draw the Lewis structure for the tellurium tetrabromide (TeBr , molecule: Video Answer Solved by verified expert Video by Dr. Satish Ingale Bharati Vidyapeeth University | Answered on 03/17/2022 Video Answers to Similar Questions Best Matched Videos Solved By Our Top Educators 01:21 BEST MATCH Draw the Lewis structure for the NH+4 N H 4 + ion.
Formula H2te Stock Illustrations – 7 Formula H2te Stock Illustrations, Vectors & Clipart – Dreamstime
6.25 pts D Question 13 Draw the Lewis structure for the tellurium tetrabromide TeBra molecule. (Note: You need to show complete solution map in your Quiz 3 Honor Pledge to receive Full credit). Edit View Insert Format Tools Table V 12pt Paragraph Β Ι Ο Α I This problem has been solved! Draw the Lewis structure for the tellurium tetrabromide (TeBr_4)molecule. – brainly.com

Source Image: brainly.com
Download Image
Lewis Structure of TeO2 (With 6 Simple Steps to Draw!) 6.25 pts D Question 13 Draw the Lewis structure for the tellurium tetrabromide TeBra molecule. (Note: You need to show complete solution map in your Quiz 3 Honor Pledge to receive Full credit). Edit View Insert Format Tools Table V 12pt Paragraph Β Ι Ο Α I This problem has been solved!
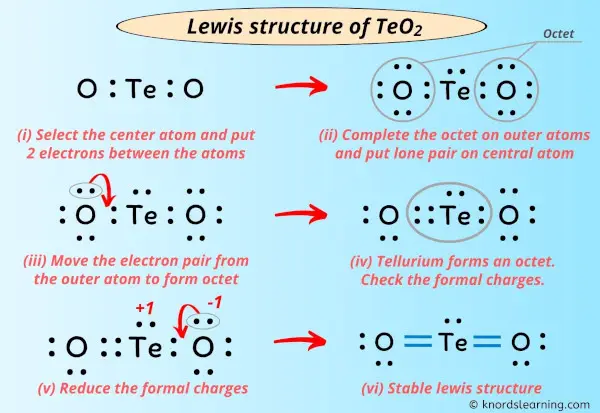
Source Image: knordslearning.com
Download Image
TeBr4 Lewis Structure in 5 Steps (With Images) For the Te structure use the periodic table to find the total number of valence electrons for the Te molecule. Once we know how many valence electrons there
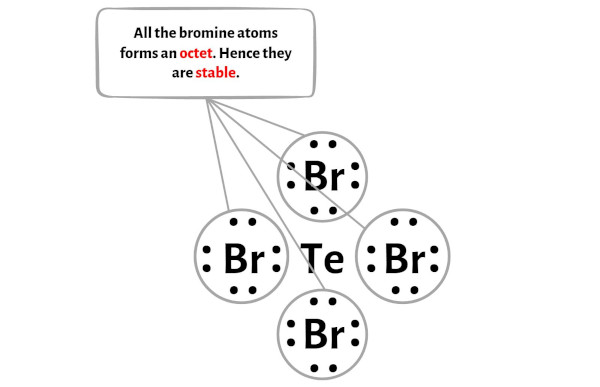
Source Image: pediabay.com
Download Image
Tellurium tetrabromide | TeBr4 | CID 82311 – PubChem Mar 13, 2023Step #1: Calculate the total number of valence electrons Here, the given molecule is TeBr4. In order to draw the lewis structure of TeBr4, first of all you have to find the total number of valence electrons present in the TeBr4 molecule. (Valence electrons are the number of electrons present in the outermost shell of an atom).
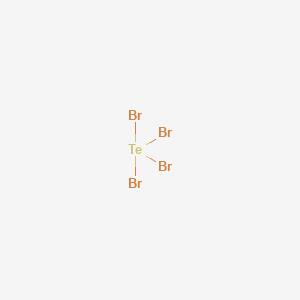
Source Image: pubchem.ncbi.nlm.nih.gov
Download Image
TeBr4 Lewis structure – Learnool Structure, properties, spectra, suppliers and links for: Tellurium tetrabromide, 10031-27-3. Jump to main content Jump to site nav. Home; … Tellurium tetrabromide. Molecular Formula Br 4 Te; Average mass 447.216 Da; Monoisotopic mass 445.579529 Da; ChemSpider ID 74282; More details: Systematic name. Tetrabromo-lambda~4~-tellane.
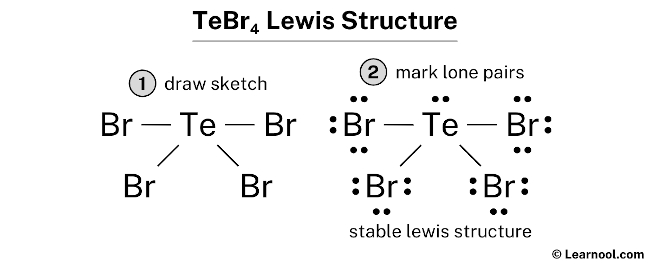
Source Image: learnool.com
Download Image
How to Draw the Lewis Dot Structure for TeBr2: Tellurium dibromide – YouTube Tellurium tetrabromide ( Te Br 4) is an inorganic chemical compound. It has a similar tetrameric structure to TeCl 4. [3] It can be made by reacting bromine and tellurium. [4] In the vapour TeBr 4 dissociates: [3] TeBr 4 → TeBr 2 + Br 2 It is a conductor when molten, dissociating into the ions TeBr 3+ and Br −.

Source Image: youtube.com
Download Image
Tellurium Tetrafluoride: Most Up-to-Date Encyclopedia, News & Reviews Draw the Lewis structure for the tellurium tetrabromide (TeBr , molecule: Video Answer Solved by verified expert Video by Dr. Satish Ingale Bharati Vidyapeeth University | Answered on 03/17/2022 Video Answers to Similar Questions Best Matched Videos Solved By Our Top Educators 01:21 BEST MATCH Draw the Lewis structure for the NH+4 N H 4 + ion.

Source Image: academic-accelerator.com
Download Image
Lewis Structure of TeO2 (With 6 Simple Steps to Draw!)
Tellurium Tetrafluoride: Most Up-to-Date Encyclopedia, News & Reviews Jun 23, 2023TeBr4 lewis structure has a Tellurium atom (Te) at the center which is surrounded by four Bromine atoms (Br). There are 4 single bonds between the Tellurium atom (Te) and each Bromine atom (Br). There is 1 lone pair on the Tellurium atom (Te) and 3 lone pairs on all the four Bromine atoms (Br).
Tellurium tetrabromide | TeBr4 | CID 82311 – PubChem How to Draw the Lewis Dot Structure for TeBr2: Tellurium dibromide – YouTube Structure, properties, spectra, suppliers and links for: Tellurium tetrabromide, 10031-27-3. Jump to main content Jump to site nav. Home; … Tellurium tetrabromide. Molecular Formula Br 4 Te; Average mass 447.216 Da; Monoisotopic mass 445.579529 Da; ChemSpider ID 74282; More details: Systematic name. Tetrabromo-lambda~4~-tellane.